Do Calcium and Rubidium Form an Ionic Compound
When you drop an ionic compound in water these water magnets will gather around it trying to pull the positive and negative ions apart. A strong base is a fully ionic base that is completely dissociated in a aqueous solution--such as water.
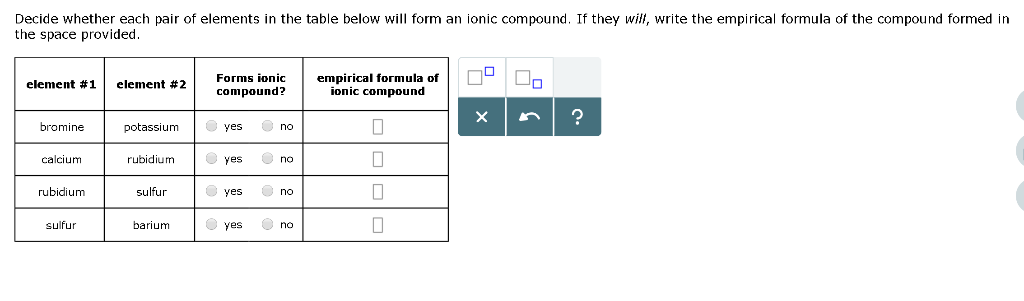
Solved Decide Whether Each Pair Of Elements In The Table Chegg Com
Mercury has been used in manufacturing as well as in dental and medical equipment fertilizers and pesticides.
. Hard water is the term used for water with a high proportion of calcium and magnesium 2 plus ions. What is the net charge of the ionic compound rubidium sulfide. Phosphoric acid H P04 and lithium hydroxide react to form a salt and water.
If the metal in the ionic compound is a transition element with variable charge metal ions the ionic charge is written as a Roman numeral following the element name of the cation. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride both white solids. Sodium fluoride dissolves easily in water but calcium fluoride does not.
The calcium usually enters the water as it flows past either calcium carbonate from limestone and chalk or calcium sulfate from other mineral deposits. One end has a positive charge while the other has a negative. Oxygen and calcium Do not form ionic bonds.
The reaction in which bigger cations stabilizes bigger anions is. Whilst some people do not like the taste hard water is generally not harmful to your health. The alkali metals consist of the chemical elements lithium Li sodium Na potassium K rubidium Rb caesium Cs and francium Fr.
Fluorine also combines with hydrogen to make hydrogen. This glossary of chemistry terms is a list of terms and definitions relevant to chemistry including chemical laws diagrams and formulae laboratory tools glassware and equipmentChemistry is a physical science concerned with the composition structure and properties of matter as well as the changes it undergoes during chemical reactions. For each of the following reactions write chemical complete ionic and net ionic equations.
Potassium rubidium and caesium. The sum of the oxidation states within a compound or ion must equal the overall charge. The conjugate bases of very weak acids.
Chemical compound any substance composed of identical molecules consisting of atoms of two or more chemical elements. What is the net charge of the ionic compound calcium fluoride. CQSOq Mg cd Z 16.
Rubidium Express your answer as an ion. Some ionic compounds arent stuck together very. Write a reaction to show that bigger cations stabilize bigger anions.
Name the alkali metals that form superoxides when heated in excess of air. If an element gains an electron will it form a positive ion or a negative ion. It features an extensive vocabulary and a.
Together with hydrogen they constitute group 1 which lies in the s-block of the periodic tableAll alkali metals have their outermost electron in an s-orbital. Fluorides hydrogen fluoride and fluorine are chemically related. Strontium Hydroxide SrOH₂.
The oxidation state of an atom is a measure of the degree of oxidation of an atom. This shared electron configuration results in their having very similar characteristic. A weak base can be defined as a chemical compound that does not fully dissociate in an aqueous solution or it can be said that the protonation in a weak base is always incomplete.
Uncombined elements have an oxidation state of 0. Cs aq OH. The small amount of compound that dissolves dissociates into ions but most of the compound remains a solid.
Rb aq OH-aq cesium hydroxide. It is characterized as a heavy silvery-white metallic liquid at room temperature that is odorless. What is the charge of a particle having 12 protons and 10 electrons.
When solutions of magnesium sul ate and calcium chloride are mixed calcium sulfate precipitates. The alkali metals that form superoxides when heated with excess air are. Sodium and magnesium nitrogen and bromine.
All the matter in the universe is composed of the atoms of more than 100 different chemical elements which are found both in pure form and combined in chemical compoundsA sample of any given pure element is composed only of. Mercury is a naturally occurring trace metalloid element and known neurotoxin with atomic symbol Hg atomic number 80 and atomic weight 20059. Fluorine is a naturally-occurring pale yellow-green gas with a sharp odor.
It is defined as being the charge that an atom would have if all bonds were ionic. Water molecules H 2 O have an unusual structure which makes them similar to a magnet.
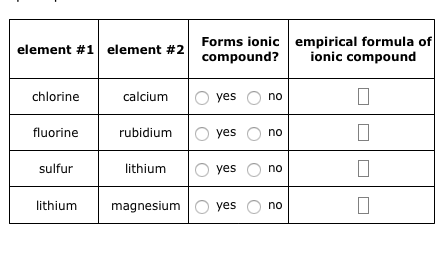
Solved Element 1 Element 2 Forms Ionic Compound Empirical Chegg Com
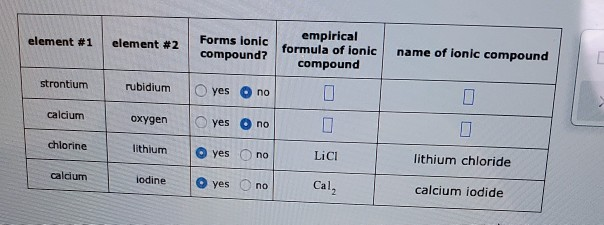
Solved Decide Whether Each Pair Of Elements In The Table Chegg Com
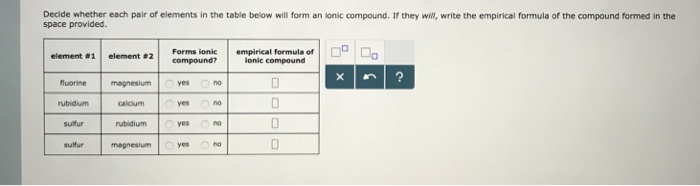
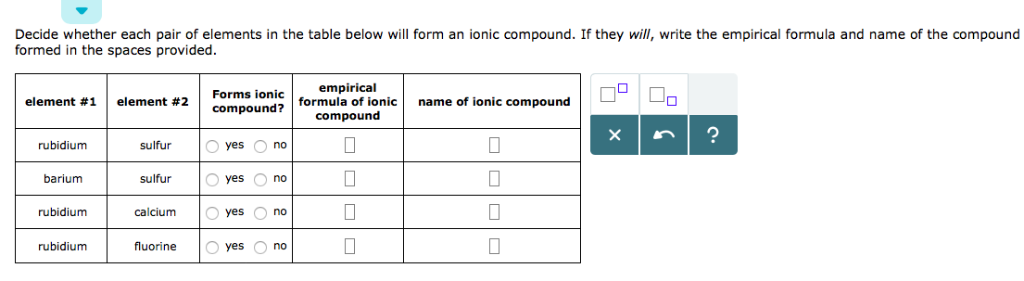
Solved Decide Whether Each Pair Of Elements In The Table Chegg Com

Solved 11 Element 21 Compound Forms Ionic Empirical Chegg Com
0 Response to "Do Calcium and Rubidium Form an Ionic Compound"
Post a Comment